The Problem
Bone implants often fail to fully integrate with surrounding tissue, limiting their effectiveness in regeneration.
Bone implants often fail to fully integrate with surrounding tissue, limiting their effectiveness in regeneration.
Using implant surface structures to deform cell nuclei and trigger secretion of proteins that promote tissue growth.
This approach enhances communication between cells and their environment, improving bone regeneration and implant success.
Professor Guillermo Ameer, Research Assistant Professor Xinlong Wang
A natural but often overlooked cellular process could hold the key to better outcomes in bone repair, according to new research from Northwestern Engineering.
A team led by Professor Guillermo Ameer discovered that when a cell’s nucleus changes shape — a process called nuclear deformation — the body boosts its ability to regenerate bone tissue, offering potential benefits for patients recovering from fractures or undergoing bone graft procedures. The pluses could include faster healing from breaks, improved outcomes for older adults, better integration of implants, and less reliance on donor grafts.
“This study expands our understanding of nuclear deformation and demonstrates its potential to enhance bone tissue regeneration — an advancement with significant implications for public health,” Ameer said.

Ameer is the Daniel Hale Williams Professor of Biomedical Engineering and Surgery at Northwestern. The inaugural director of the Querrey Simpson Institute for Regenerative Engineering at Northwestern University (QSI RENU), and the Center for Advanced Regenerative Engineering (CARE), Ameer is a leader in regenerative engineering, biomaterials, additive manufacturing for biomedical devices, controlled drug delivery, and bio/nanotechnology for therapeutics.
The team found that when cell nuclei are physically deformed, the cells secrete proteins that organize the extracellular matrix (ECM) — the structure that supports and signals to surrounding cells. These secretions, in turn, promote the transformation of nearby human mesenchymal stromal cells (hMSCs) into bone-forming cells.
With Xinlong Wang, a research assistant professor in QSI RENU, Ameer reported the latest findings in “Microtopography-Induced Changes in Cell Nucleus Morphology Enhance Bone Regeneration by Modulating the Cellular Secretome,” published July 11 in Nature Communications.
The researchers validated their findings in a mouse model with critical-size cranial defects. When hMSCs with deformed nuclei were placed on structures designed to mimic bone, the cells increased expression of a gene involved in bone matrix production. This shift helped lead to more robust bone formation.
The results are a step toward shaping the future of implant design and bone regeneration therapies. The collection of factors a cell secretes, its secretome, is an emerging focus in modern medicine, especially in cell-based therapies. By showing how nuclear deformation alters the secretome, this research adds an important layer of understanding to how engineered materials interact with living tissue.
These findings could be used to inform the design and fabrication of bioactive implants for bone regeneration, with potential applications in spinal fusion, dental implants, craniofacial reconstruction, and the treatment of large bone defects. By enhancing how implants interact with surrounding tissue, the approach could improve healing outcomes and reduce complications in procedures that depend on effective bone integration.
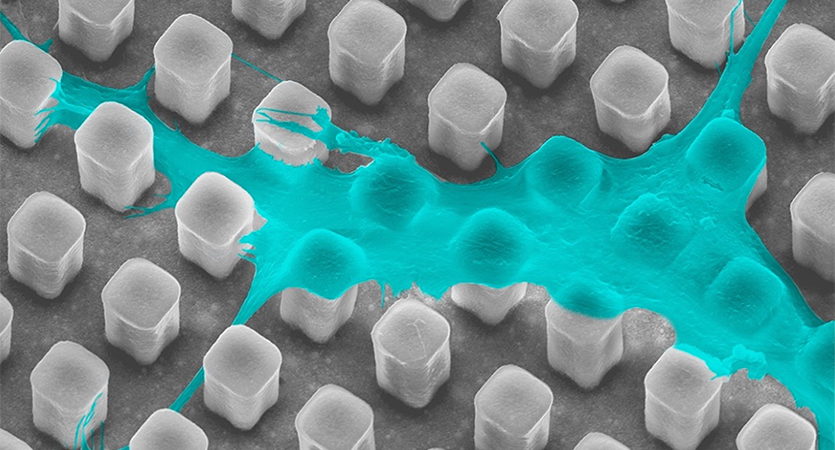
“By performing experiments both in vitro and in vivo, we have a clearer understanding of how the relationship between nuclear deformation and the cell secretome can improve bone regeneration,” Wang said. “These findings could be used to inform the design and fabrication of bioactive implants for bone regeneration [via control of nuclear deformation and the resulting cellular secretome].”
While many previous efforts in regenerative engineering have focused on how biomaterials physically affect cells that contact them, this work could broaden the scope to include how the affected cells then influence their environment.
“Most previous studies have focused on the direct interactions between biomaterials and deformed cells – in cell culture systems in the lab,” Ameer said. “In contrast, this study explores how deformed cells influence their surrounding cellular environment in the body, an important and fundamental question in regenerative engineering.”
This paper continues earlier research that used micropillar implants to regrow bone tissue. That work showed promise but challenges remain: how to accelerate bone formation and limited understanding of cell-ECM interactions that lead to bone formation. To address these gaps, the team added hydroxyapatite, a key component of natural bone, and studied the impact of the cell secretome in more detail.
“In the future, we aim to develop technologies capable of fabricating 3D scaffolds with micropillar topography to enable the regeneration of larger volumes of tissue,” Ameer said.